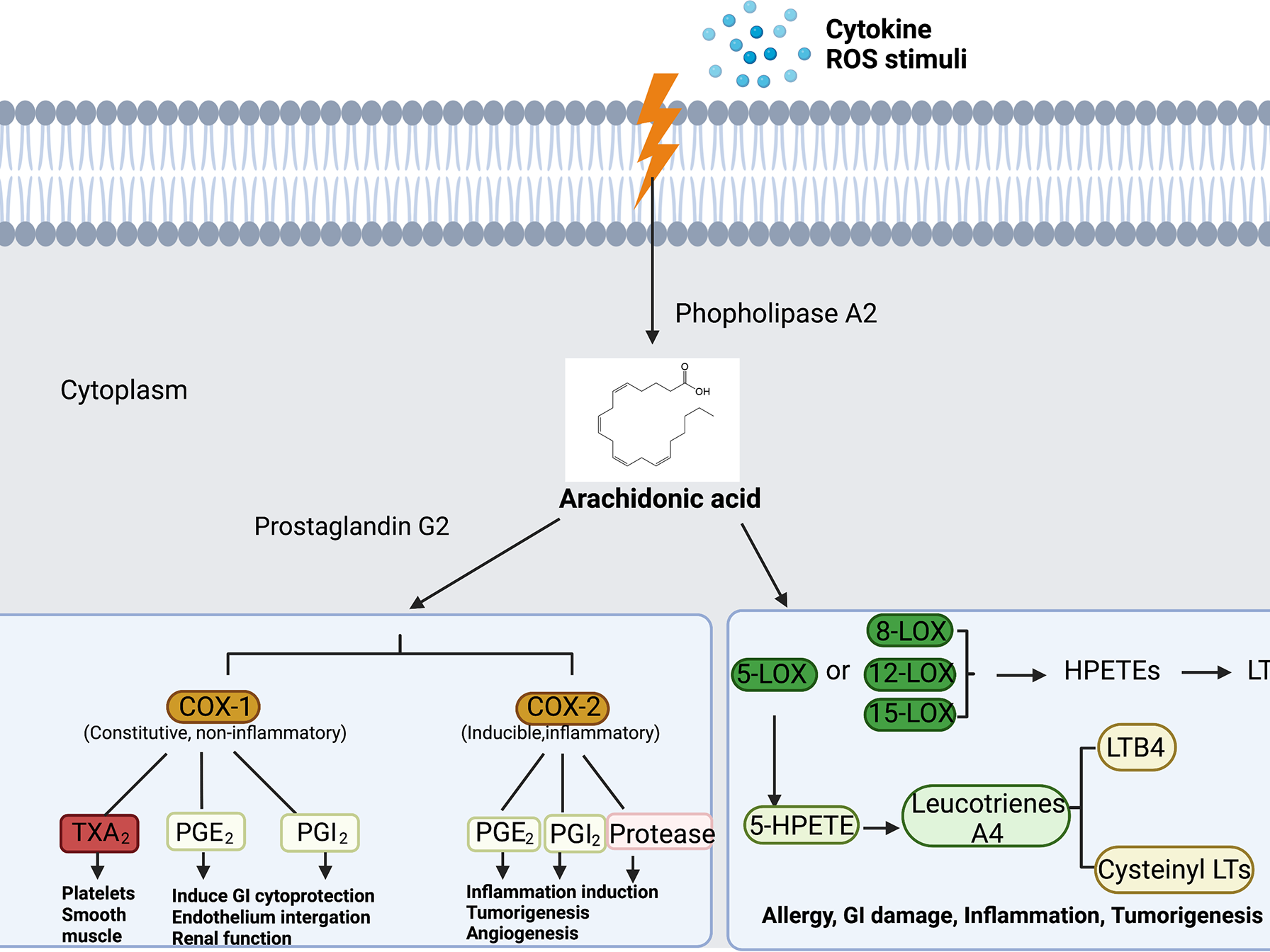
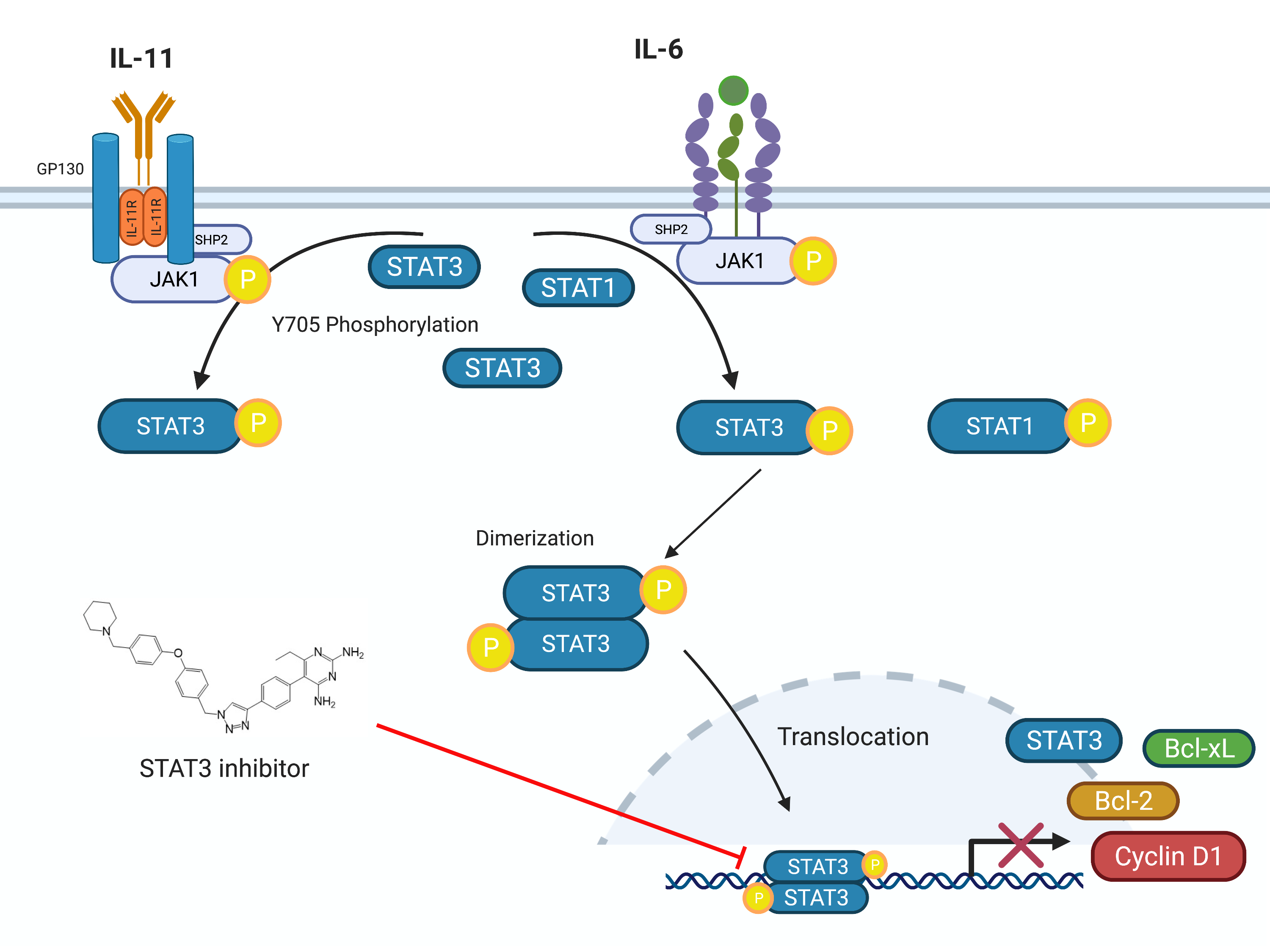
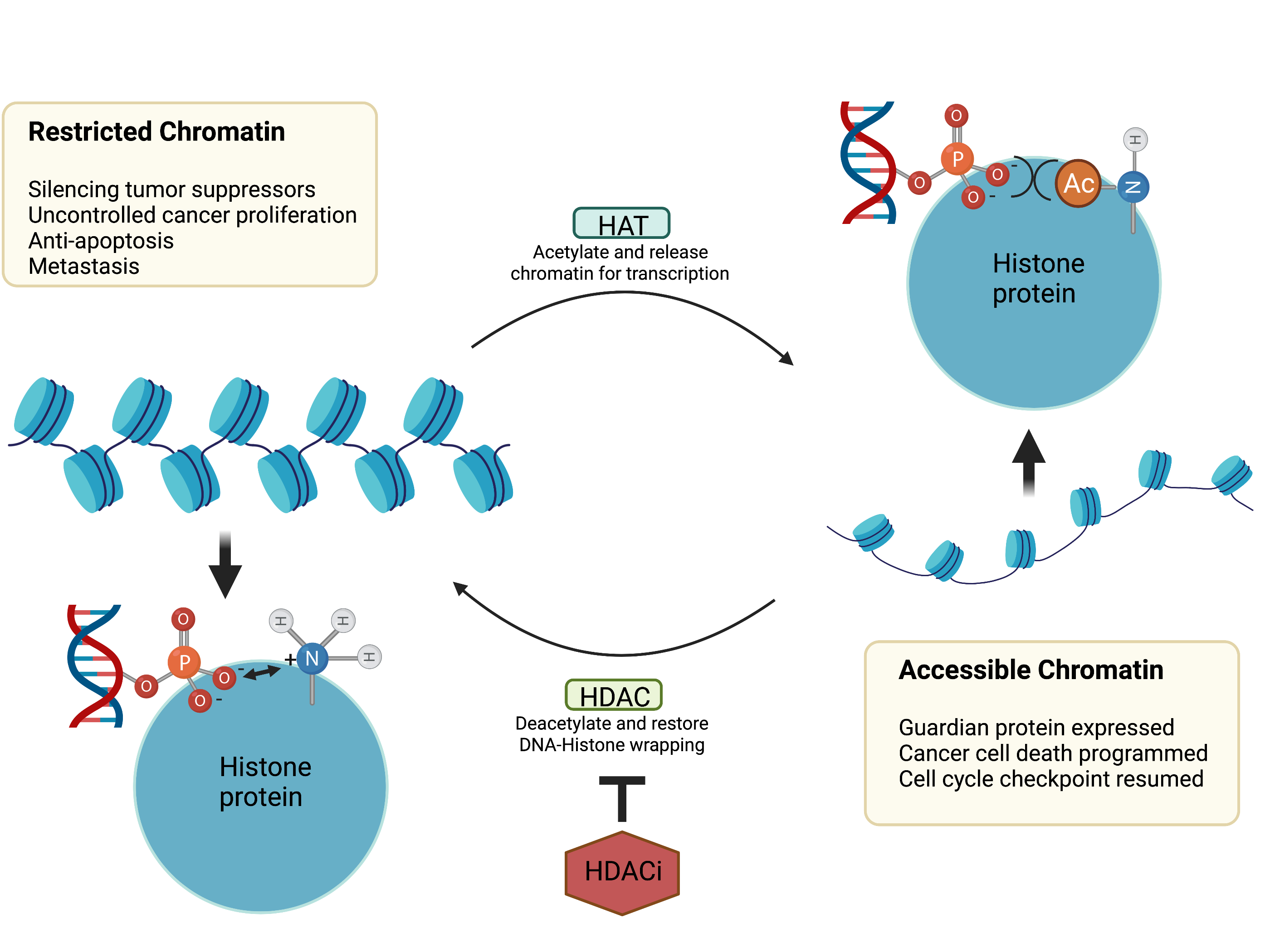
Inflammation is like the proverbial double-edged sword. Controlled short-term inflammation could be a protective response while uncontrolled inflammation sustains multiple disease types, including several chronic diseases and cancers. The aim of this review article was to provide an in-depth analysis of the relationships and distinctions between uncontrolled inflammation, fibrosis and cancers. Herein, we emphasized the challenges and opportunities of developing novel therapies for the treatment and/or management of these diseases. We described how the therapeutic benefits of current agents could be enhanced through drug delivery systems, combination therapy and the integration of tissue-targeted and/or pathways selective strategies. In the pursuit of the next generation of safer therapies, we also postulated on the value of the re-evaluation of the disease-specific roles of multiple pathways implicated in the development and sustenance of chronic inflammatory diseases and cancers as well as the application of single-cell screening technologies in the discovery of novel disease-relevant proteins for the more precise targeting of these diseases.
Inhibitors for Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic and fatal disease that progressively declines the lung function. The FDA approved drugs - pirfenidone (PFD) and nintedanib – are suboptimal in the management of PFD largely due to their toxic side effects, low potency, cost ineffectiveness and minimal beneficial effect on the patients’ survival rate. We described herein four classes of macrolide-based anti-fibrotic agents designed, using a structure-based approach, from PFD and azithromycin and clarithromycin as macrolide templates. These agents are designed to exploit the excellent PK and selective lungs and/or liver tissues distribution activities of these macrolide templates to arrive at novel anti-fibrotic agents that may selectively accumulate within these tissues. We synthesized 80 compounds and tested their effects on the viability of four cell lines –MRC-5, PCS-201-012, PCS-201-020, PCS-300-010, PCS-301-010, A549 Hep-G2, VERO, MDA-MB-231, MCF-7, LnCap, and DU145 . We observed that compounds 10c, 11c, 11b, 15c, 20e inhibited the proliferation of these cell lines with IC50 range of 2.5-10μM. Additionally, these compounds potently inhibit NF-κB and TGF-β pathways through the luciferase reporter gene screening. The dose-dependent curves of TGF-β inhibition assay show enhanced potency as high as 1000-fold relative to PFD or the unmodified macrolide templates. Compounds 15c showed an optimum inhibition and/or downregulation of the fibrosis markers (FN-1, MMP-9, COL1A1, α-SMA) that we investigated at low micromolar IC50. The next best compounds are 10c, 11c and 20e. Collectively, these compounds are excellent candidates for future preclinical studies focused on the evaluation of their potential as tissue-selective anti-fibrotic effects in in vivo models of IPF and liver fibrosis.